- ACRYSTEX MS RESIN
- AMILAN™ PA
- BIFFA r-HDPE
- BIFFA r-PP COMPOUND
- CELANEX® PBT
- CELANYL® PA
- CLEARFLEX LLDPE
- CRASTIN
- DELRIN
- DIAKON PMMA
- DISTRUPOL-PRECW
- DRYFLEX CIRCULAR
- DRYFLEX TPE
- ECOMID® PA
- EGYEUROPTENE
- ELASTOLLAN TPU
- ELTEX MED
- ELTEX P POLYPROPYLENE
- ELVAKON PMMA
- ENTHOVEN RECYCLED PPC
- ERACLENE HDPE
- EVICOM PVC COMPOUNDS
- FLEXIRENE LLDPE
- FRIANYL® PA
- GREENFLEX EVA
- HY-VIN PVC COMPOUNDS
- HYTREL
- INEOS EBA
- INEOS EMAA
- INEOS LDPE
- INEOS LLDPE
- INEOS PP
- INEOS mLLDPE
- IPETHENE LDPE
- KIBILAC ASA
- KIBISAN SAN
- KIBITON Q-RESIN
- KIBITON TPE
- LG ABS
- LG ASA
- LG LUPOX PC/PBT
- LG LUPOY PC
- LG LUPOY PC/ABS
- LG LUPOY PC/ASA
- LG LUSEP PPS
- LG SAN
- MEDALIST TPE
- MEDIPRENE TPE
- MINLON PA
- MONPRENE TPE
- PEARLENE
- PHARMALENE PE & EVA
- POLYLAC ABS
- POLYREX PS
- PURGEX CLEANING COMPOUND
- PVOH (Polyvinyl Alcohol)
- RECYCL-IN
- RIBLENE LDPE
- RIGIDEX HDPE
- RIGIDEX MDPE
- RYNITE PET
- SARLINK TPV AND TPE
- THERMOFIL REINFORCED PP COMPOUNDS
- TORAYCON PBT
- TORELINA PPS
- TOYOLAC ABS
- TOYOLAC MABS
- TRIFILON BIOLITE
- TRIFILON REVO
- TRIFILON SWITCH
- TUFCOAT PMMA
- WELLS PLASTIC ANTISTATICS
- WELLS PLASTICS ANTIBLOCKS
- WELLS PLASTICS ANTIMICROBIALS
- WELLS PLASTICS ANTIOXIDANTS
- WELLS PLASTICS BLOWING AGENTS
- WELLS PLASTICS FLAME RETARDANT MASTERBATCHES
- WELLS PLASTICS FRAGRANCES
- WELLS PLASTICS OXO BIODEGRADABLES
- WELLS PLASTICS PROCESSING AIDS
- WELLS PLASTICS PURGE AND CLEANING COMPOUNDS
- WELLS PLASTICS SLIP AGENTS
- WELLS PLASTICS UV STABILISERS
- WONDERLITE PC
- WONDERLOY PC/ABS
- ZYTEL PLUS
- ZYTEL HTN
- ZYTEL LCPA
- ZYTEL PA
- ZYTEL RS
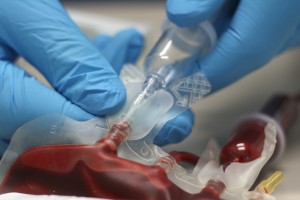
Distrupol delivers high-quality thermoplastics to the healthcare industry. These thermoplastics are used in the manufacture of demanding components across many different healthcare segments.

Range:
Distrupol draws on our longstanding experience in materials, application development, technology, safety and regulatory compliance to provide expert support to healthcare product manufacturers, backed by our global polymer supply partners. Depending on the specific application, Distrupol can deliver an appropriate solution from our broad range of standard products, or from our portfolio of medically approved grades, which are differentiated by a greater degree of testing, manufacturing control and regulatory support.
Support:
We can help select a 'fit for purpose' solution for your applications, recommending the most suitable materials. We understand the numerous requirements and considerations specific to the medical industry, enabling you to get it right first time. Our development engineers can also help you with conceptual design, mould flow and tooling, material sampling and process optimisation.
Quality, certification, traceability and confidence:
Authenticity and traceability of materials being used for your components is critical. We can supply you with certificates of conformity and analysis with every delivery, and give you full traceability and the confidence that you, and your moulders, are using the right material that is certified and in specification.
|
|

Sterilisation Methods
Autoclave Steam Sterilisation
Steam sterilisation, or autoclaving, is a widely used method. It is comparatively easy to control (environmentally friendly) and can be performed with relatively low-cost equipment. An autoclave combines heat and moisture at elevated pressures. Heat sterilisation of medical instruments or components with the presence of moisture significantly speeds up heat penetration (steam sterilisation). The time and temperature for sterilisation depends on pressure and the type of microorganisms to be inactivated. Typical process conditions are 20 min at 121°C or 5 min at 134°C.
Ethylene Oxide (EtO) Sterilisation
EtO sterilisation is the gaseous method of sterilisation involving the highly diffusive, permeable and toxic EtO gas. The use of EtO sterilisation evolved for sterilising heat and moisture-sensitive medical devices and or devices containing electronic components. The processes of EtO sterilisation involve several stages of gas removal: humidification, EtO exposure and air washes. Process pressures are close to vacuum and temperatures used are circa 50°C. Many plastic materials are compatible with EtO sterilisation.
Plasma Sterilisation
Plasma is a gas sterilisation method where different gases can be used, but Hydrogen Peroxide is the most common. Plasma can be considered were an alternative to EtO is required or the high sterilisation temperatures of Autoclave are not suitable. The plasma sterilisation process is safe and easy to use and is typically used for devices that cannot be sterilised with high heat.
Gamma Ray Sterilisation
Sterilisation by gamma radiation uses the radioisotope Cobalt 60 as its energy source. Items for sterilisation pass the radiation field where gamma rays pass readily through plastics and kill bacteria by breaking the covalent bonds of bacterial DNA. They are measured in units called kiloGrays (kGy). There is no heat or moisture generated during the process and consequently, there is no resulting heat stress and condensate drainage. Most importantly, there is no residual radioactivity after irradiation.
Electron Beam Sterilisation
The electron beam sterilisation process begins with an electron beam accelerator to create a powerful beam of electrons. The beam is scanned back and forth to create a curtain of fast electrons ensuring a uniform dose of radiation to objects passing the beam. E-beam sterilisation destroys all types of pathogens including viruses, fungi, bacteria, parasites, spores, and molds.
ISO 10993 – Explained
The International Organization for Standardization (ISO) was established to determine uniform worldwide standards. ISO developed a standard for biological evaluation of medical devices - ISO 10993 - in 1995, which is a 20-part standard used to evaluate the effects of medical devices and their component materials on the body.
The most influential guideline for biocompatibility is the first part of this standard, “ISO 10993- Part 1: Evaluation and Testing,” which provides a methodology for choosing the proper biological evaluation test program.
From here it is possible to determine also which test program to utilise depending on the device category of which there are three: Surface, External Communicating and Implant, and the exposure period of the material: Limited (<24 hours), Prolonged (24 hours to 30 days) and Permanent (>30 days). Typically ISO 10993-Part 5 is the most relevant to polymers covering cytotoxicity similar to USP monograph <87>.
| ISO Part | Title |
|---|---|
| 1 | Evaluation and testing |
| 2 | Animal welfare requirements |
| 3 | Tests for genotoxicity, carcinogenicity and reproductive toxicity |
| 4 | Selection of tests for interactions with blood |
| 5 | Tests for in vitro cytotoxicity |
| 6 | Tests for local effects after implantation |
| 7 | Ethylene oxide sterilisation residuals |
| 8 | Clinical investigation of medical devices |
| 9 | Framework for identification and quantification of potential degradation products |
| 10 | Tests for irritation and delayed type hypersensitivity |
| 11 | Tests for systemic toxicity |
| 12 | Sample preparation and reference materials |
| 13 | Identification and quantification of degradation products from polymeric medical devices |
| 14 | Identification and quantification of degradation products from ceramics |
| 15 | Identification and quantification of degradation products from metals and alloys |
| 16 | Toxicokinetic study design for degradation products and leachables |
| 17 | Establishment of allowable limits for leachable substances |
| 18 | Chemical characterisation of materials |
| 19 | Physico-chemical, morphological and topographical characterisation of materials |
| 20 | Principles and methods for immunotoxicology testing of medical devices |
ISO 10993 Device Categories
| Device Categories | Body Contact | Examples |
|---|---|---|
| Surface Devices | Skin |
Electrodes, external prostheses, fixation tapes, compression bandages, monitors of various types |
| Mucous Membrane |
Contact lenses, urinary catheters, intravaginal and intraintestinal devices (stomach tubes, sigmoidoscopes, colonoscopes, gastroscopes), endotracheal tubes, bronchoscopes, dental prostheses, orthodontic devices, IUDs |
|
| Breached or Compomised Surfaces |
Ulcer, burn and granulation tissue dressings or healing devices, occlusive patches |
|
| External Communication Devices |
Blood Path Indirect |
Solution administration sets, extension sets, transfer sets, blood administration sets |
| Tissue/ Bone/Dentin Communicating |
Laparoscopes, arthroscopes, draining systems, dental cements, dental filling materials, skin staples |
|
| Circulation |
Intravascular catheters, temporary pacemaker electrodes, oxygenators, extracorporeal oxygenator tubing and accessories, dialysers, dialysis tubing and accessories, hemoadsorbents, immunoadsorbents |
|
| Implant Devices |
Tissue/Bone Implant Devices |
Orthopedic pins, plates, replacement joints, bone prostheses, cement and intraosseous devices, pacemakers, drug supply devices, neuromuscular sensors and simulators, replacement tendons, breast implants, artificial larynxes, subperiostealimplants, ligation clips |
|
Blood |
Pacemaker electrodes, artificial arteriovenous fistulae, heart valves, vascular grafts, internal drug delivery catheters, ventricular assist devices |
Medical & Biocompatibility Tests
What is Biocompatibility?
It is a material’s lack of interaction with living tissue or a living system by not being toxic, injurious, or physiologically reactive, and not causing immunological rejection.
Ultimately, a material’s chemical components will not cause the patient harm. No single test may be sufficient to define biocompatibility.
Two common test regimens are used to measure biocompatibility, United States Pharmacopeia (USP) and International Organisation for Standardisation (ISO), tests for biological evaluation of medical devices.
What is USP Class VI?
The United States Pharmacopeia (USP) is an independent organisation that established a set of standards to ensure the quality of medicines and health care technologies. USP protocols are used to classify plastics in Classes I - VI, based on end use, type and time of exposure of human tissue to plastics, of which Class VI requires the most stringent testing of all the six classes.
- Systemic toxicity tests are used to determine the irritant effect of toxic leachables present in extracts of test materials.
- Intracutaneous tests are used to assess the localised reaction of tissue to leachable substances.
- Implantation tests are used to evaluate the reaction of living tissue to the plastic.
Product range
Useful information
Select a resource from the drop down to find out more:
Looking for datasheets & UL yellow cards?
Select the trade name and grade below:
- ACRYSTEX MS RESIN
- AMILAN™ PA
- BIFFA r-HDPE
- BIFFA r-PP COMPOUND
- CELANEX® PBT
- CELANYL® PA
- CLEARFLEX LLDPE
- CRASTIN
- DELRIN
- DIAKON PMMA
- DISTRUPOL-PRECW
- DRYFLEX CIRCULAR
- DRYFLEX TPE
- ECOMID® PA
- EGYEUROPTENE
- ELASTOLLAN TPU
- ELTEX MED
- ELTEX P POLYPROPYLENE
- ELVAKON PMMA
- ENTHOVEN RECYCLED PPC
- ERACLENE HDPE
- EVICOM PVC COMPOUNDS
- FLEXIRENE LLDPE
- FRIANYL® PA
- GREENFLEX EVA
- HY-VIN PVC COMPOUNDS
- HYTREL
- INEOS EBA
- INEOS EMAA
- INEOS LDPE
- INEOS LLDPE
- INEOS PP
- INEOS mLLDPE
- IPETHENE LDPE
- KIBILAC ASA
- KIBISAN SAN
- KIBITON Q-RESIN
- KIBITON TPE
- LG ABS
- LG ASA
- LG LUPOX PC/PBT
- LG LUPOY PC
- LG LUPOY PC/ABS
- LG LUPOY PC/ASA
- LG LUSEP PPS
- LG SAN
- MEDALIST TPE
- MEDIPRENE TPE
- MINLON PA
- MONPRENE TPE
- PEARLENE
- PHARMALENE PE & EVA
- POLYLAC ABS
- POLYREX PS
- PURGEX CLEANING COMPOUND
- PVOH (Polyvinyl Alcohol)
- RECYCL-IN
- RIBLENE LDPE
- RIGIDEX HDPE
- RIGIDEX MDPE
- RYNITE PET
- SARLINK TPV AND TPE
- THERMOFIL REINFORCED PP COMPOUNDS
- TORAYCON PBT
- TORELINA PPS
- TOYOLAC ABS
- TOYOLAC MABS
- TRIFILON BIOLITE
- TRIFILON REVO
- TRIFILON SWITCH
- TUFCOAT PMMA
- WELLS PLASTIC ANTISTATICS
- WELLS PLASTICS ANTIBLOCKS
- WELLS PLASTICS ANTIMICROBIALS
- WELLS PLASTICS ANTIOXIDANTS
- WELLS PLASTICS BLOWING AGENTS
- WELLS PLASTICS FLAME RETARDANT MASTERBATCHES
- WELLS PLASTICS FRAGRANCES
- WELLS PLASTICS OXO BIODEGRADABLES
- WELLS PLASTICS PROCESSING AIDS
- WELLS PLASTICS PURGE AND CLEANING COMPOUNDS
- WELLS PLASTICS SLIP AGENTS
- WELLS PLASTICS UV STABILISERS
- WONDERLITE PC
- WONDERLOY PC/ABS
- ZYTEL PLUS
- ZYTEL HTN
- ZYTEL LCPA
- ZYTEL PA
- ZYTEL RS
Technical questions?
Click here to complete a form and a member of the technical team will be in touch shortly.
Need a quote?
Click here to complete a short form and a team member will be in touch shortly.
© 2023 Distrupol. All rights reserved.





